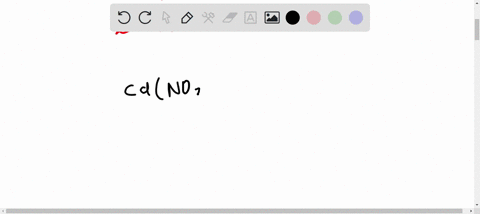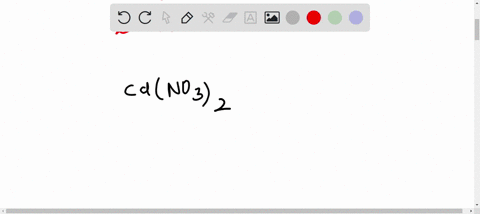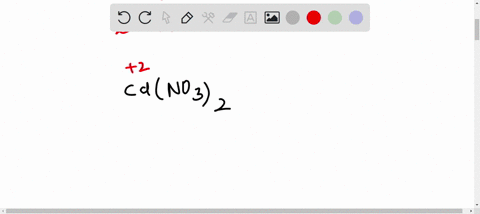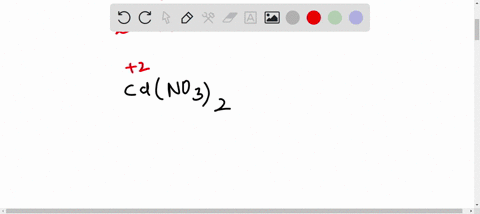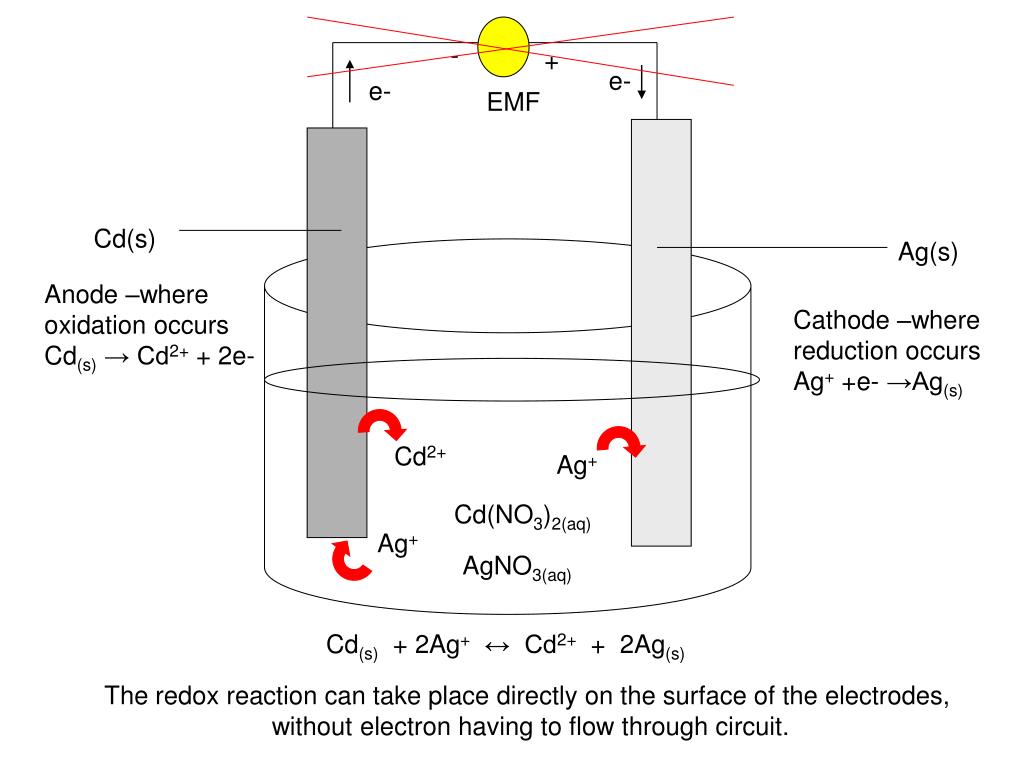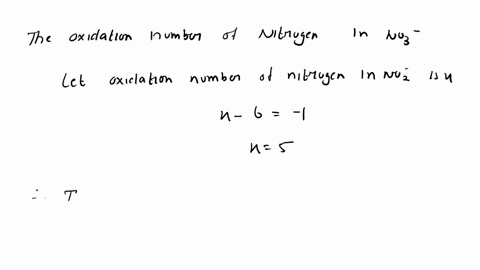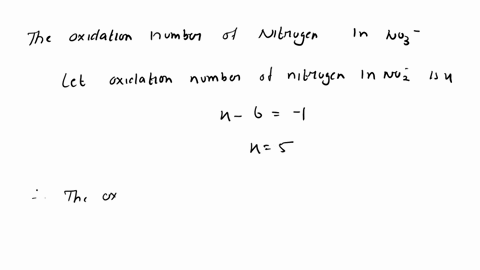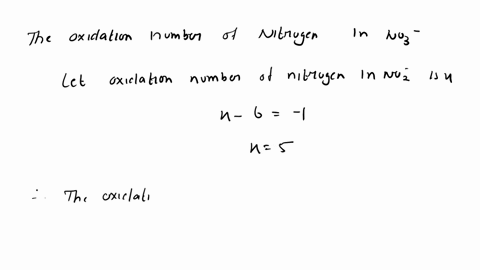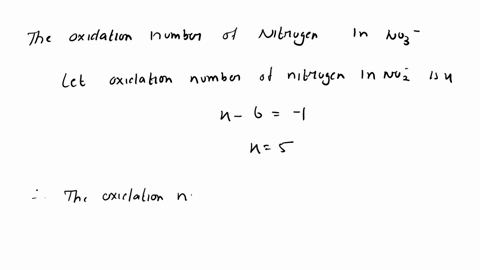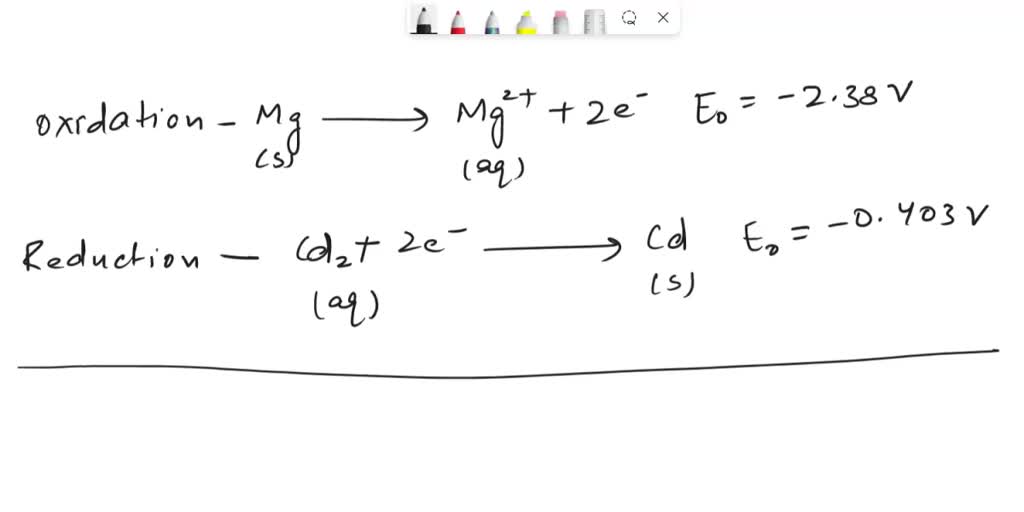
SOLVED: A Galvanic cell consists of Mg electrode in a 1.0 mol Mg(NO3)2 solution and a Cd electrode in a 1.0 mol a Cd(NO3)2 solution. determine the cell reaction and calculate the
![Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect](https://ars.els-cdn.com/content/image/1-s2.0-S1463018400000241-gr3.gif)
Reaction of Cd(NO3)2·4H2O with 4,4'–bipyridine (bpy) in MeOH solvent: synthesis and characterization of T-shaped [Cd(bpy)1.5(NO3)2]·3H2O, square grid [Cd(bpy)2(H2O)2](NO3)2·4H2O and linear polymeric [Cd(bpy)(H2O)2(NO3)2] - ScienceDirect

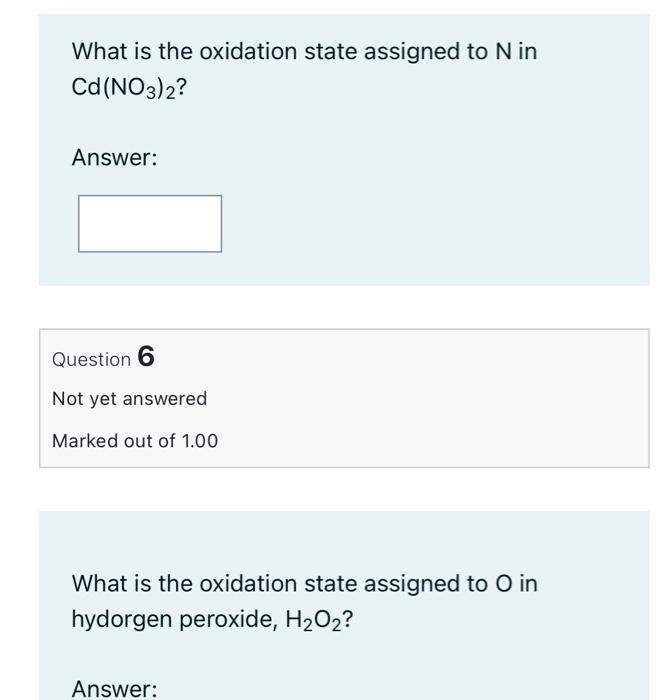
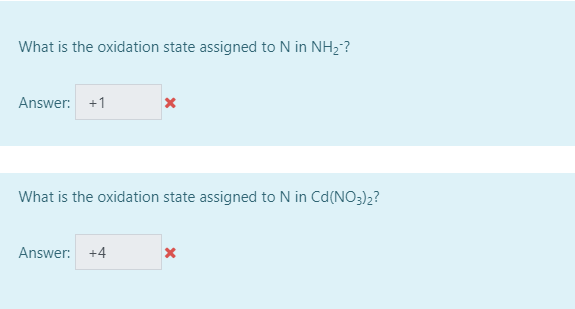
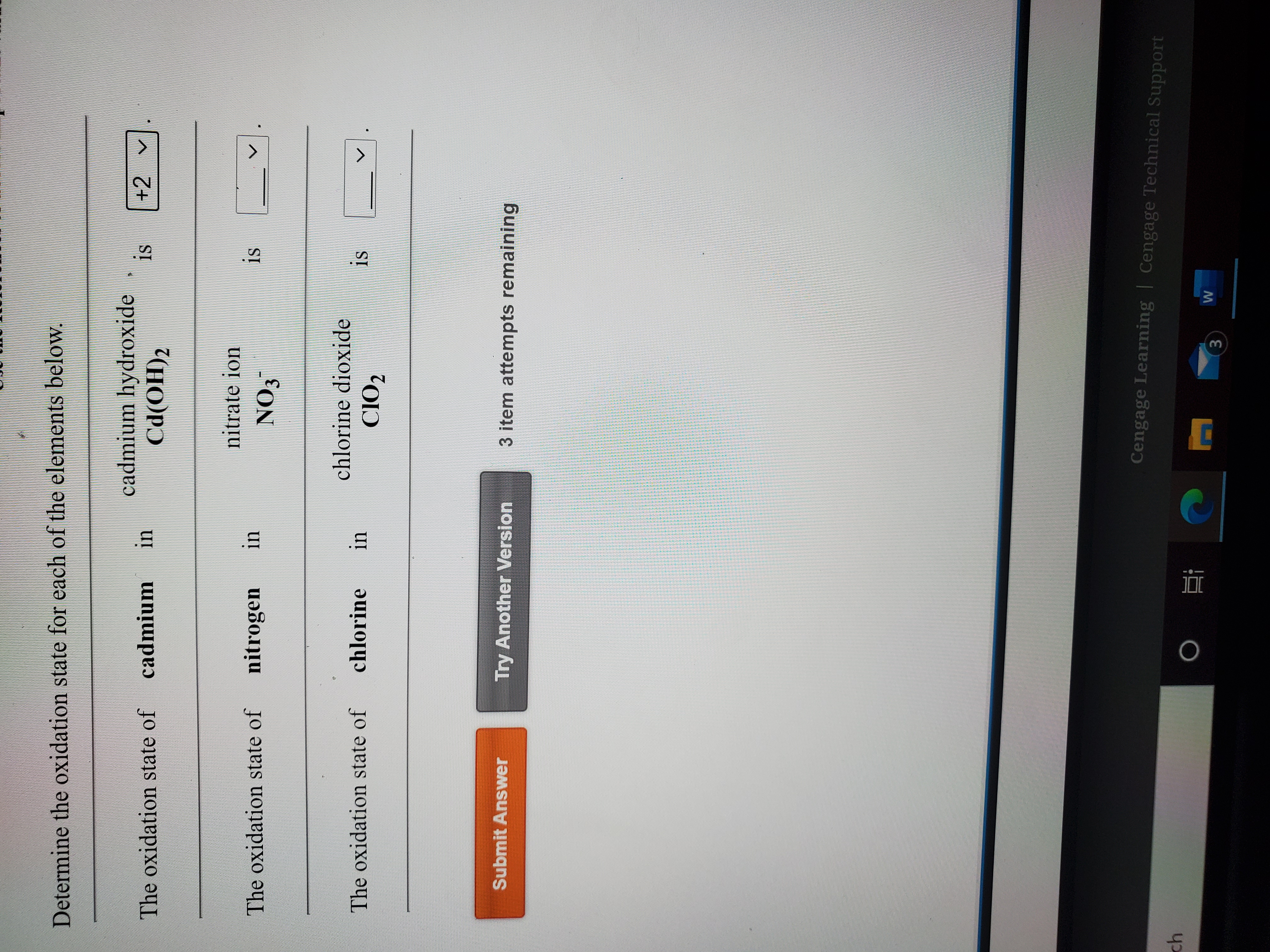
SOLVED: Determine the oxidation number of each element in each of the following: HSO3- b. NaClO2 Cu(OH)2 KMnO4 SnCl2 Na3PO4 Cu2+ Au3+ B. Identify the reactant being oxidized and the one being
![What is the oxidation number of the metal in each of the following complexes: (a) (CO(NH3). Cl2 (b) (COSO (NH3)4]NOZ [CD(SCN)]2+ (Cr(en)3]C13 (e) CuCl2(CH3NH2)2] (f) (AIH) (g) [Fe(CN)614- (h) (OsCI N12- ( What is the oxidation number of the metal in each of the following complexes: (a) (CO(NH3). Cl2 (b) (COSO (NH3)4]NOZ [CD(SCN)]2+ (Cr(en)3]C13 (e) CuCl2(CH3NH2)2] (f) (AIH) (g) [Fe(CN)614- (h) (OsCI N12- (](https://toppr-doubts-media.s3.amazonaws.com/images/10616518/a4fb32a9-4c56-43bd-bbe0-f3b9fa2bef28.jpg)